Following the norm: Usability documentation
Developing for your users
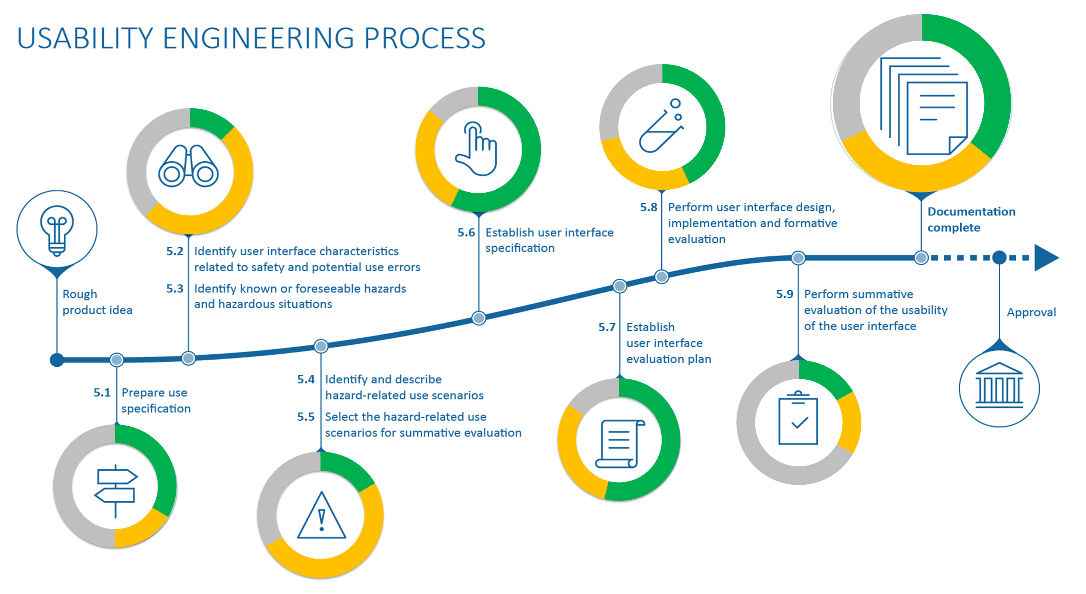
Use-Lab has experience creating and evaluating complete usability documentation, such as Use Scenarios, Use Specifications or the complete Usability Engineering File. Just ask us – we can review your existing documents or create your documentation from scratch in close consultation with you. We take into account the relevant laws and standards such as MDR, IEC 62366, ISO 14971 and ISO 13485.

Use-Lab is a reliable, flexible and creative partner for usability studies in the context of combination product and medical device development.
Florian Schauderna
Senior Manager Usability/Human Factors Engineering
Leave nothing to chance
With more than 20 years of experience working towards the approval of medical devices and documentation of the associated processes, as well as active participation in numerous national and international standardization bodies and working groups, Use-Lab is a competent partner in the preparation of approval-relevant documents.
Of course, we discuss the individual requirements and the international target markets with each customer in advance. We create all documents accompanying the development, such as the specification documents or evaluation plans and reports, in compliance with the standards, so that they can be directly linked to the usability engineering file. In addition, we are happy to take over the creation of the usability engineering file or the human factors evaluation report for the FDA or support you in the creation of the risk management file, the design documentation or the final product file.
With more than 20 years of experience working towards the approval of medical devices and documentation of the associated processes, as well as active participation in numerous national and international standardization bodies and working groups, Use-Lab is a competent partner in the preparation of approval-relevant documents.
Of course, we discuss the individual requirements and the international target markets with each customer in advance. We create all documents accompanying the development, such as the specification documents or evaluation plans and reports, in compliance with the standards, so that they can be directly linked to the usability engineering file. In addition, we are happy to take over the creation of the usability engineering file or the human factors evaluation report for the FDA or support you in the creation of the risk management file, the design documentation or the final product file.



